Responsabilidades esenciales del Departamento de Calidad en la búsqueda del éxito organizacional
Feb 26,2024
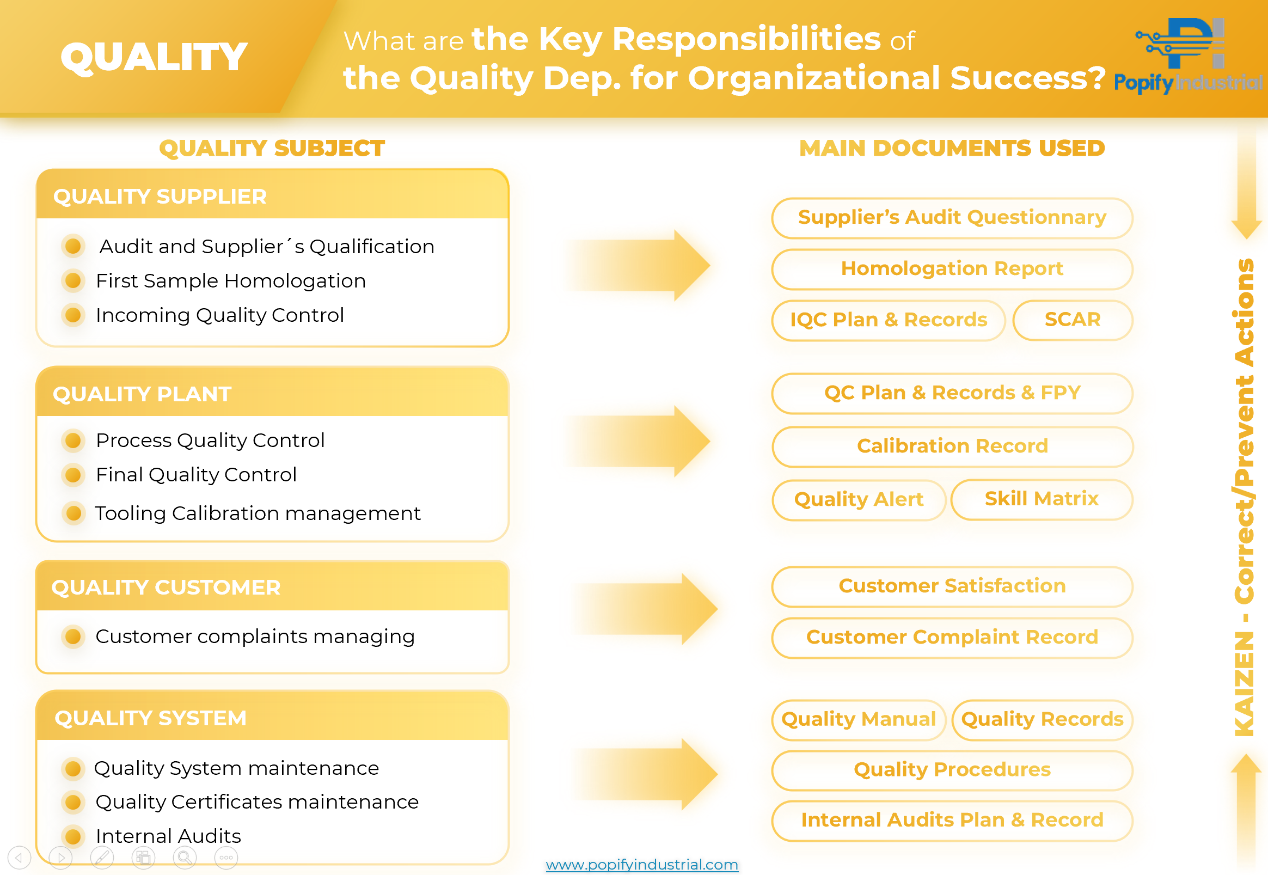
Essential Responsibilities of the Quality Department in Pursuing Organizational Success
In today's industrial reality, Quality is a promise and a commitment to every customer. The Quality Department is the custodian of this promise, implementing systems and processes that ensure each product meets or exceeds expectations. From supplier evaluation to customer satisfaction and the maintenance of quality systems, each responsibility is carried out with precision and meticulous attention.
1️. Supplier Quality: The First Line of Defense.
Before engaging in business relations, the Quality Department must conduct a rigorous audit of suppliers. Using a detailed Audit Questionnaire, they must evaluate compliance with defined quality standards. The Homologation of First Samples and the Incoming Quality Control are also crucial steps to ensure that only materials that meet the defined criteria are accepted into the supply chain, avoiding potential failures from the start.
- Key Documents:
- Supplier Audit Questionnaire
- Homologation Report
- Incoming Quality Control Plans and Records (IQC)
- Supplier Corrective Action Report (SCAR)
2️. In-Plant Quality Control: Maintaining Process Integrity.
Within the plant, quality must be constantly monitored. From Process Quality Control to Final Quality Control, each component and every stage of the process are inspected to maintain product integrity.
- Key Documents:
- Quality Control Plans and Records (QC)
- Final Product Yield (FPY)
- Calibration Records
- Quality Alert
- Skills Matrix
3️. Customer Quality: Listen, Resolve, and Improve.
Managing customer complaints is a priority for the Quality Department. Incidents are not only resolved, but they are also analyzed to improve processes and products. Customer satisfaction is a key indicator of our success.
- Key Documents:
- Customer Satisfaction Record
- Customer Complaints Record
4️. System Quality: The Mark of Excellence
Possessing the right Quality Certifications is more than a badge; it's a commitment to excellence. These certifications are passports to global markets, proving compliance with international standards. Maintaining these certifications requires a symphony of documentation and procedures, all meticulously managed and updated. Furthermore, the regular conduction of internal audits is critical to evaluate and improve our processes.
- Key Documents
- Quality Manual
- Quality Records
- Quality Procedures
- Internal Audit Plan and Record
⚠ Furthermore, across all the aforementioned areas, we should employ robust Corrective & Preventive Action Tools, such as the 8D method. It ensures not only the effective resolution of any issues that arise but also the implementation of measures to prevent their recurrence, underscoring our unwavering dedication to continuous improvement and unsurpassed quality.
📞 If you're looking for solutions that align with excellence in quality and wish to learn more about how our products or services can meet your specific expectations, contact@popifyindustrial.com and let us show you what quality means from our perspective.
Noticias relacionadas


